The tools of theoretical chemistry can be used to virtually any problem in chemistry. The major focus of our group is the understanding of the interaction between photons and molecules. Physicists are nowadays able to control light in time, energy, or position with unprecedented precision. Our goal is to understand how we can control the molecules via the light. At the same time, we aim to model molecules beyond the gas phase, investigating finite molecular aggregates or molecules in the condensed phase. The ongoing projects in the Slavíček group are:
X-ray initiated photodynamics and spectroscopy

Photochemistry typically concentrates on the interaction of UV or visible light with molecules, forcing the valence electrons to move. The X-ray photons have much higher energy, being able to excite or ionize the inner shell electrons. These excitations are specific that means we can sensitively choose which atom in a molecule will be excited. It opens up enormous possibilities for applications, e.g. targeted release of biologically active molecules. The ultrafast processes following the core or inner valence electron excitation are, however, only poorly understood. We model these reactions for solvated molecules to give a molecular level insight to the field of radiation chemistry. The field is recently witnessing exciting progress; new phenomena have recently emerged, such as Intermolecular Coulomb Decay that allows for energy transfer between neighboring molecules. We expect that these processes will become a basis for new types of spectroscopies. Furthermore, energy transfer between molecules has considerable potential in biomedical applications.
Atmospheric chemistry and astrochemistry
Atmosphere of the Earth at first sight seems somewhat boring for a chemist: there is not much chemistry one can do with nitrogen and oxygen. But the opposite is true. Processes are triggered by sun light and the atmosphere works as a rather rich chemical reactor. The chemical and photochemical reactions often take place on liquid or solid aerosols rather than in the gas phase; a good example are processes on polar stratospheric clouds related to ozone depletion. The assistance of theoretical chemistry in the field of atmospheric chemistry is vital as many of the key species are unstable or poorly defined (e.g. aerosols). We model the electronic spectra of atmospheric aerosols as well the photodynamical processes following the excitation of the molecules. Our studies naturally expand to the field of astrochemistry.
Biomolecular photostability
Photons are bringing an enormous amount of energy to molecules. It is therefore expected that a molecule hit by a photon will fragment or react with another molecule. Photostability means that this extra energy rapidly transforms into heat, which is later dissipated via the interaction with the environment, and the molecule survives. The question of photostability is especially important for “molecules of life”, such as nucleic acids or proteins since their vulnerability to the UV light might severely limit the lifetime of these molecules. In our laboratory, we investigate the photostability of various heterocycles and of proteins, focusing on the role solvent molecules.
Electron transfer processes and ionization in aqueous systems
Electron transfer belongs among the most significant processes in all fields of chemistry and biochemistry. We simulate redox processes in systems which are difficult to access experimentally, e.g. unstable species such as solvated electron or radicals. We are also interested in electron transfer processes in the interfaces. Redox processes are closely related to ionization. Photoionization is a connection between the field of ab initio electrochemistry and our major expertise, theoretical photodynamics.
Development of the computational photodynamics methods for complex systems
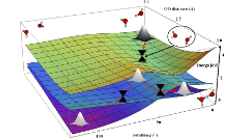
In our work, we combine methods of quantum chemistry (what are the electrons doing?), classical and quantum dynamics (what are the nuclei doing?), theoretical spectroscopy (what are the experimental observables?) and statistical mechanics (what do we see if there is a large number of molecules?). Part of our work is necessarily devoted to the development of new and more efficient techniques of molecular simulations. We develop new methods for simulating electronic spectra, new quantum chemical approaches such as FOMO-CAS-CI or novel approaches for modeling solvent (MC-VEEP method).
Applied quantum chemistry
 Part of the capacity of the laboratory is used to assist chemist in different field to guide and interpret their research. We e.g. cooperate with the group of Dr. Filip Teplý (Institute of Organic Chemistry and Biochemistry, AS CR) on the research of the helquats, novel class of compounds developed in his laboratory.
Part of the capacity of the laboratory is used to assist chemist in different field to guide and interpret their research. We e.g. cooperate with the group of Dr. Filip Teplý (Institute of Organic Chemistry and Biochemistry, AS CR) on the research of the helquats, novel class of compounds developed in his laboratory.